

Beryllium is an exception: It does not react with water or steam, and its halides are covalent. Magnesium reacts only with steam and calcium with hot water. They react readily with halogens to form ionic salts, and can react slowly with water. These metals are less active than the alkali metals, but are still fairly active. Berylium is the least metallic element in the group and tends form covalent bonds in its compounds.
Periodic table simple states plus#
These elements all have two valence electrons and tend to lose both to form ions with a two plus charge. The alkaline earth metals are silvery colored, soft, low-density metals, though are a bit harder than the alkali metals.
Periodic table simple states series#
The series consists of the elements beryllium ( Be), magnesium ( Mg), calcium ( Ca), strontium ( Sr), barium ( Ba) and radium ( Ra) (though radium is not always considered an alkaline on earth due to its radioactivity). The alkaline earth metals are the series of elements in Group 2 of the periodic table.
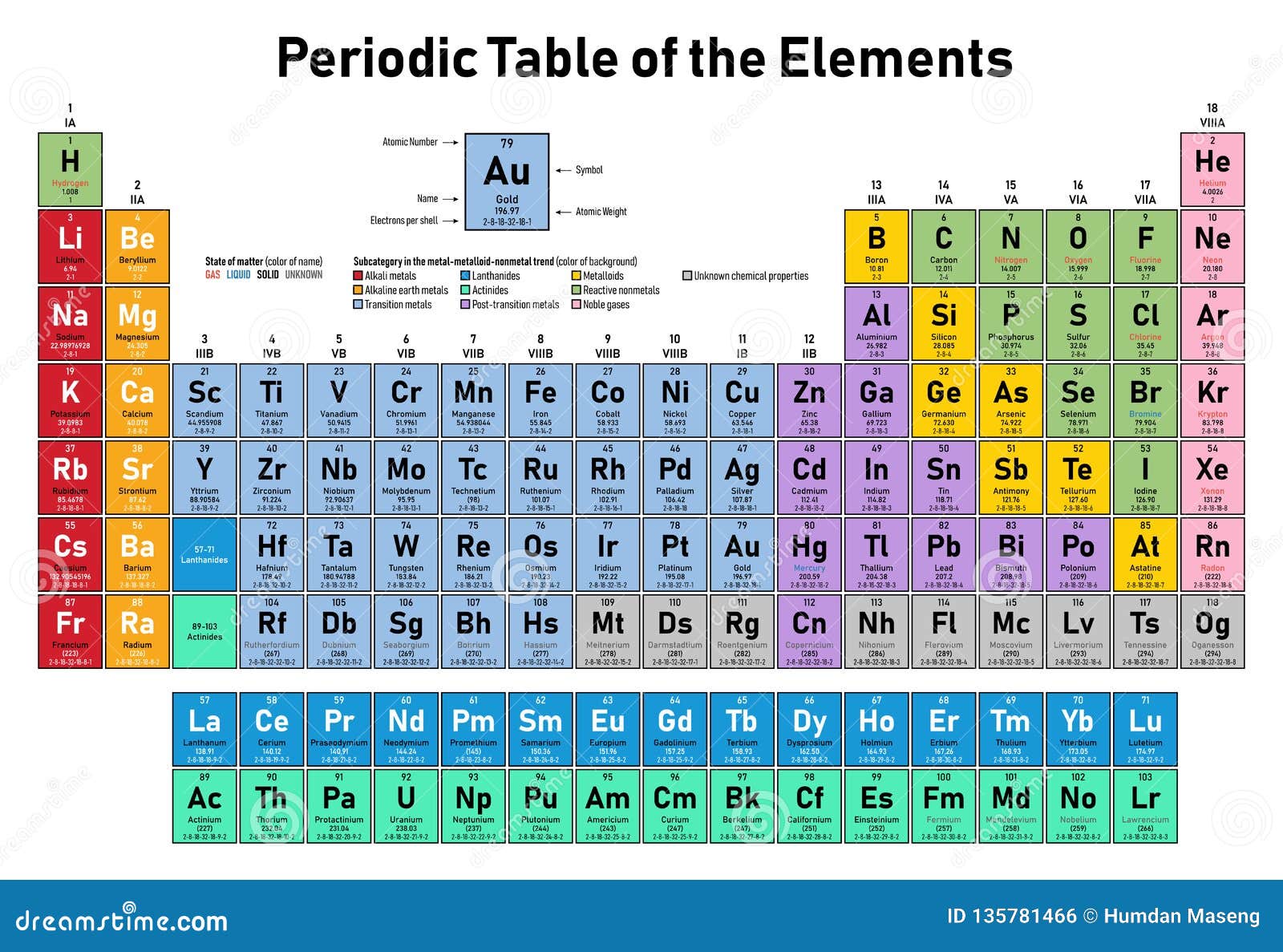
Under extremely high pressure, such as is found at the core of Jupiter, hydrogen does become metallic and behaves like an alkali metal see metallic hydrogen. In compounds hydrogen most often forms covalent bonds. The hydride ion is an extremely strong base and does not usually occur except when combined with the alkali metals and some transition metals (i.e. Unlike the alkali metals hydrogen atoms can also gain an electron to form the negatively charged hydride ion.
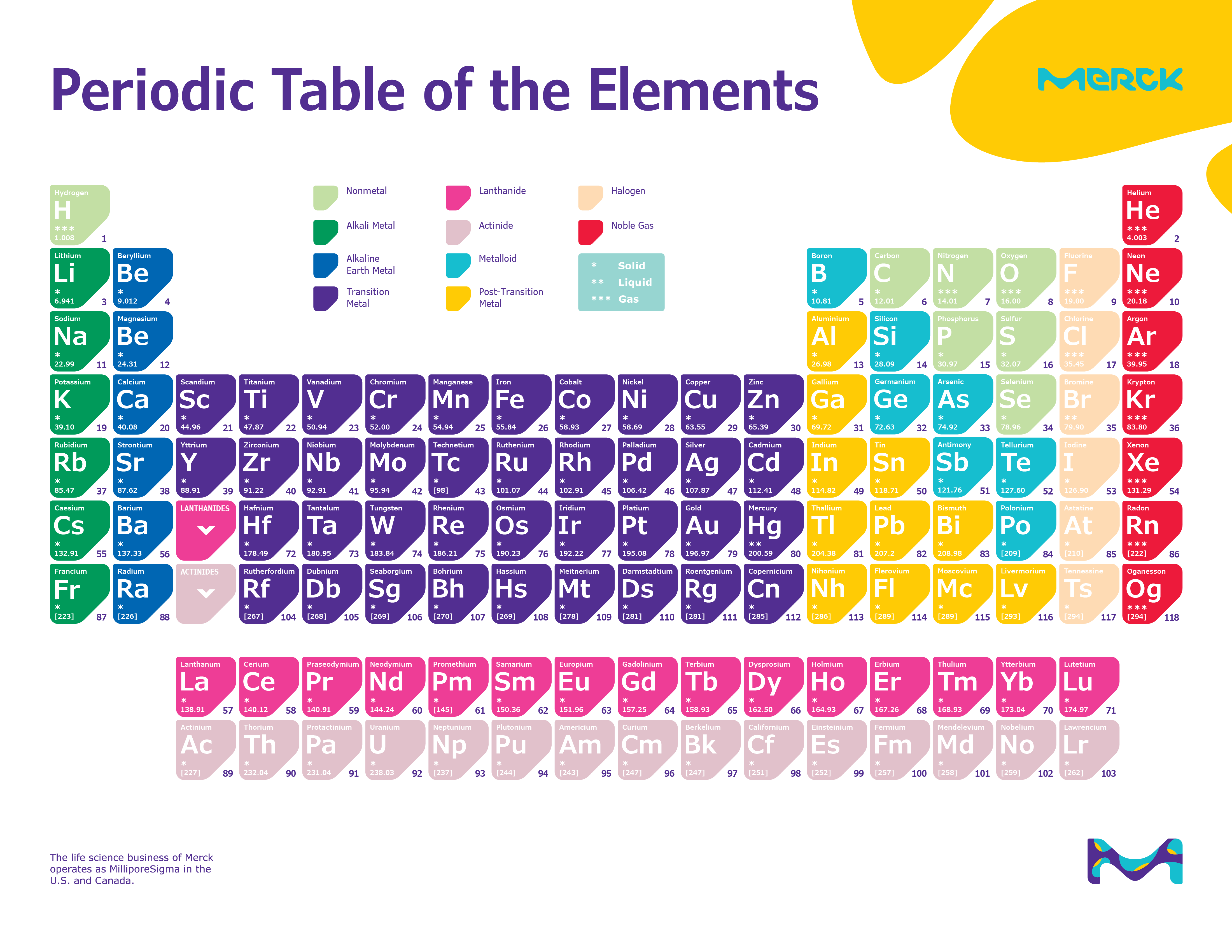
Hydrogen can form ions with a single positive charge, but removal of its single electron requires considerably more energy than removal of the outer electron from the alkali metals. Hydrogen also has a single valence electron and is usually placed at the top of Group 1, but it is not a metal (except under extreme circumstances as metallic hydrogen) rather it exists naturally as a diatomic gas. It is from this character that they derive their group name. In particular the hydoxides resulting from the reaction with water are our most common laboratory bases (alkalis). The oxides, hydrides, and hydoxides of these metals are basic (alkaline). The reaction with water is as follows:Īlkali metal + water → Alkali metal hydroxide + hydrogenĢ K ( s ) + 2 H 2 O ( l ) → 2 K O H ( a q ) + H 2 ( g ) As we move down the group the reactions become increasingly violent. These reactions also often liberate sufficient energy to ignite the hydrogen and can be quite dangerous. They are famous for their vigorous reactions with water to liberate hydrogen gas. The alkali metals react readily with halogens to form ionic salts, such as table salt, sodium chloride (NaCl). Due to their activity they occur naturally in ionic compounds not in their elemental state. This makes them very reactive and they are the most active metals. They have the lowest ionization energies in their respective periods. These elements all have one valence electron which is easily lost to form an ion with a single positive charge. The alkali metals are silver-colored (caesium has a golden tinge), soft, low- density metals. The series consists of the elements lithium ( Li), sodium ( Na), potassium ( K), rubidium ( Rb), caesium ( Cs), and francium ( Fr). The alkali metals are the series of elements in Group 1 of the periodic table (excluding hydrogen in all but one rare circumstance).


 0 kommentar(er)
0 kommentar(er)
